CODEX Alimentarius was founded in 1963 as part of the World Health Organisation Food and Agriculture Organisation (WHO/FAO) and is the international body that sets guidelines for national regulatory authorities. In 2009, they finally reached consensus upon a definition of dietary fiber after almost 20 years of deliberation:
.png)
It should be noted that the McCleary method (AOAC 2009.01/2011.25) is the only method that correctly measures total dietary fiber as currently defined by CODEX in any/all samples. For more information on this aspect of dietary fiber, please see the section on measurement of dietary fiber.
The current CODEX definition was accepted in order to 1) reflect the current body of scientific knowledge on dietary fiber, 2) to promote the concept that any and all substances that behave like fiber in vivo, regardless of their source, can be included as dietary fiber provided that physiological health benefits can be demonstrated for them and 3) to guide international harmonisation for food labelling and food composition tables.
Since the new definition has been published, many national and international authorities have moved to redefine their regulations based on this. An excellent article published in 2014 by Dr. Julie Jones1 outlines the current global situation: The European Food Safety Authority (EFSA – regulatory body for the entire European Union), Food Standards Australia and New Zealand (FSANZ), Health Canada and equivalent bodies in China, Brazil, Chile, Mexico, Thailand, Korea, Malaysia and Indonesia have all broadly accepted the CODEX definition (including carbohydrates with DP3-9 as described in footnote 1 of the definition) and the accompanying analytical method AOAC 2009.01. South Africa is currently the only country that has decided not to include oligosaccharides with DP3-9 in their definition of dietary fiber which may prove somewhat problematic in terms of regulation as there is no analytical method available to meet that definition precisely.
.png) In the USA, the FDA has proposed to also accept the CODEX definition and the accompanying analytical method AOAC 2009.01 (and to reject an analytically unmanageable definition given by the IOM in 2001). The proposed FDA rule change to 21 CFR part 1012 is expected to come into effect in 2016.
In the USA, the FDA has proposed to also accept the CODEX definition and the accompanying analytical method AOAC 2009.01 (and to reject an analytically unmanageable definition given by the IOM in 2001). The proposed FDA rule change to 21 CFR part 1012 is expected to come into effect in 2016.
The UK has traditionally been the only country to use a completely different definition that is based on the use of the non-starch polysaccharide (NSP) method to measure ‘fibre’. It is unclear at this time whether they will take this opportunity to harmonise with the rest of the world in this regard.
Megazyme is the only commercial supplier of the reagents required to run AOAC 2009.01. These are sold in the form of a kit (Integrated Total Dietary Fiber Assay Kit) which also contains a data booklet clearly outlining the principle of the method and detailed instructions for how to practically perform the procedure in the laboratory.
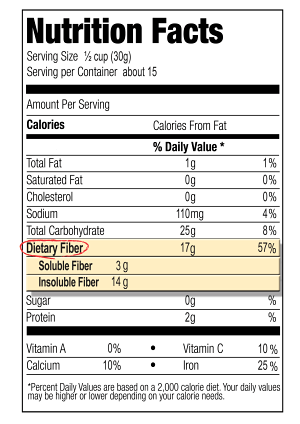
Nutrition Facts Labelling
Acceptance of the CODEX definition by a national governing body has important implications for compliance with nutrition facts labelling regulations. As AOAC 2009.01 is the only analytical method that can accurately measure dietary fiber as defined by CODEX, the onus is now placed on food manufacturers to ensure that the dietary fiber content on their product labels agrees with the values obtained with AOAC method 2009.01. This can have significant consequences for companies wishing to make specific health claims based on dietary fiber content. The rules and regulations relating to this topic can vary from country to country. The United States and European Union are examined as test cases:
United States
Dietary Fiber Nutrient Content Claims
Claims made on food product packaging need to be tightly regulated. It is important that the consumer is not misled in their assessment of how healthy a particular food product is. Nutrient content claims are dependent on three factors:
1) The daily value (DV) is the FDA’s recommended daily amount of an ingredient. The DV for dietary fiber is 25g for a 2000 calorie diet.
2) The reference amount customarily consumed (RACC) is set by the FDA and is basically the guide serving quantity of the product in question.
3) Claim thresholds are set by the FDA and are based on the percentage of the DV present in the RACC for a given product.
10-19% of DV in RACC allows “good source” claim.
20+% of DV in RACC allows “high” or “excellent” source claim.
As an example, the RACC for rice is 45g dry weight so greater than (2.5g / 45g = 5.6%) fiber content allows a food manufacturer to make good source claim.
Four separate health claims have been approved by the FDA on fiber containing products. These health claims fall under the categories:
1) Fiber-containing foods and cancer
2) Fiber-containing foods and coronary heart disease
3) Fruits and vegetables and cancer
4) Soluble fiber from certain foods and risk of coronary heart disease
There are strict guidelines governing when one of these claims can be made for a given product and the wording that can be used in such a claim. The rules are quite complex and are outlined in full here. As an example, an allowable health claim under category 1 above would be:
Upcoming changes
The rule change proposed by the FDA that is expected to come into effect in 20162 will see significant changes with respect to regulations relating to dietary fiber. First and foremost, on acceptance of the CODEX definition and the accompanying analytical method AOAC 2009.01, FDA will begin performing spot-checks on food products that are currently on the market. FDA’s own analytical labs will perform AOAC 2009.01 for the determination of total dietary fiber in a food sample and compare the values obtained to those quoted on the nutrition facts label. Discrepancies will be fully investigated with the food manufacturer.
The concept that isolated or synthetic non-digestible carbohydrates added to foods can only have their fiber content included in the ‘Total Dietary Fiber’ value if that isolated or synthetic non-digestible carbohydrate has been shown to have a beneficial physiological effect, is also proposed in the current rule change. If no beneficial physiological effect has been demonstrated, then food manufacturer’s in-house record keeping would be used to subtract the fiber content of the isolated or synthetic non-digestible carbohydrate from the ‘Total Dietary Fiber’ value determined using AOAC 2009.01. Food manufacturers will be able to apply to the FDA via a petition (as per 21 CFR 10.302) with reliable scientific evidence outlining the beneficial physiological effects for any isolated or synthetic non-digestible carbohydrate that they wish to be permissible as ‘dietary fiber’. The FDA will make judgement on all such petitions.
The European Food Safety Authority (EFSA) panel on Dietetic Products, Nutrition and Allergies recently published a scientific opinion on dietary reference values for carbohydrates and dietary fiber at the request of the European Commision (EC).3 This opinion recommends acceptance of the CODEX definition of dietary fiber and the accompanying analytical method AOAC 2009.01 and also recommends a daily allowance of 25 g of dietary fiber for an adult. The European Commission has also accepted the CODEX definition and AOAC 2009.01 as the most suitable analytical method for this definition and this has been included in Annex 1 of Regulation 1169/2011/EU on the provision of food information to consumers. Regulation 1169/2011/EU will apply from 13 December 2014 and at the same time Directive 90/496/EEC will be repealed.4
Dietary Fiber Nutrient Content Claims
According to EFSA, a food can be referred to as a “source of fibre” or “containing fibre”, provided that the product contains at least 3 g/100 g or 1.5 g/100 kcal of dietary fibre. Correspondingly, a food can be referred to as “high in fibre” or “containing a large amount of fibre”, provided that the product contains at least 6 g/100 g or 3 g/100 kcal of dietary fibre.
Dietary Fiber Health Claims
All health claims in the EU are covered by the Nutrition and Health Claims Regulation (NHCR). This includes all commercial presentations (product labels, print media, spoken or video presentations, company websites and social media). Health claims are split into four categories outlined below and are the evaluation of every claim is carried out by EFSA with final authorisation coming from the EC.
The EFSA panel on Dietetic Products, Nutrition, and Allergies published a scientific opinion in 2010 after investigating the link between dietary fiber consumption and a wide range of health benefits including satiety, weight management, normal blood glucose concentrations, normal blood cholesterol concentrations, normal bowel function and regularity, reduction of postprandial glycaemic response, decreasing potentially pathogenic gastro-intestinal microorganisms, increasing the number of gastrointestinal microorganisms and fat absorption. Their judgement at that time was that there was insufficient evidence to warrant an official health claim for dietary fiber (as a general food ingredient) for any of these physiological effects,5 however, seven health claims for individual fiber components have been successfully approved separately. A table detailing these successful health claims is shown here. Over time, substantiated health claims will undoubtedly continue to appear on the EU Register of Nutrition and Health Claims in response to applications made by food manufacturers.